Effect of isouronium/guanidinium substitution on the efficacy of a series of novel anti-cancer agents†
Abstract
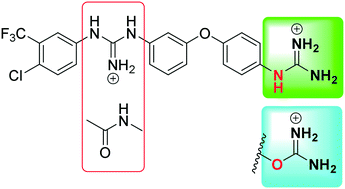
Considering our hypothesis that the guanidinium moiety in the protein kinase type III inhibitor 1 interacts with a phosphate of ATP within the hinge region, the nature of the interactions established between a model isouronium and the phosphate groups of ATP was computationally analysed indicating that an isouronium derivative of 1 will interact in a similar manner with ATP. Thus, a number of compounds were prepared to assess the effect of the guanidinium/isouronium substitution on cancer cell growth; additionally, the molecular shortening and conformational change induced by replacing the di-substituted guanidine-linker of 1 by an amide was explored. The effect of these compounds on cell viability was tested in human leukaemia, breast cancer and cervical cancer cell lines and the resulting IC50 values were compared with those of the lead compound 1. Replacement of the di-substituted guanidine-linker by an amide results in the loss of cytotoxicity; however, substitution of the mono-substituted guanidinium by an isouronium cation seems to be beneficial for cell growth inhibition. Additionally, the effect of these compounds on the MAPK/ERK pathway was studied by means of Western blotting and the results indicate that the isouronium derivative 2 decreases the levels of phosphorylated, and thus activated, ERK (pERK) both in leukaemia and breast cancer cells, whereas lead compound 1 only shows an effect on pERK levels in breast cancer cells. This confirms that both compounds could interfere with the MAPK/ERK pathway although other targets cannot be ruled out.


 Please wait while we load your content...
Please wait while we load your content...