Human stomach-on-a-chip with luminal flow and peristaltic-like motility†
Abstract
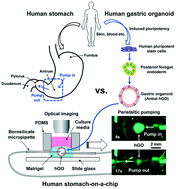
Current in vitro approaches and animal models have critical limitations for modeling human gastrointestinal diseases because they may not properly represent multicellular human primary tissues. Therefore, there is a need for model platforms that recapitulate human in vivo development, physiology, and disease processes to validate new therapeutics. One of the major steps toward this goal was the generation of three-dimensional (3D) human gastric organoids (hGOs) via the directed differentiation of human pluripotent stem cells (hPSCs). The normal functions and diseases of the stomach occur in the luminal epithelium, however accessing the epithelium on the inside of organoids is challenging. We sought to develop a bioengineered platform to introduce luminal flow through hGOs to better model in vivo gastric functions. Here, we report an innovative microfluidic imaging platform housing hGOs with peristaltic luminal flow in vitro. This human stomach-on-a-chip allows robust, long-term, 3D growth of hGOs with the capacity for luminal delivery via a peristaltic pump. Organoids were cannulated and medium containing fluorescent dextran was delivered through the lumen using a peristaltic pump. This system also allowed us to rhythmically introduce stretch and contraction to the organoid, reminiscent of gastric motility. Our platform has the potential for long-term delivery of nutrients or pharmacological agents into the gastric lumen in vitro for the study of human gastric physiology, disease modeling, and drug screening, among other possibilities.
- This article is part of the themed collection: Organ-, body- and disease-on-a-chip systems


 Please wait while we load your content...
Please wait while we load your content...
