Na2S2O8-mediated efficient synthesis of isothiocyanates from primary amines in water†
Abstract
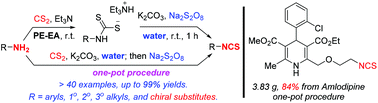
We have developed two green, practical, and efficient procedures, including a one-pot one, to synthesize isothiocyanates from amines and carbon disulfide via desulfurization with sodium persulfate. Water is used as the solvent. Basic conditions are necessary for good chemoselectivity for isothiocyanates. Structurally diverse linear and branched alkyl amines and aryl amines are readily converted to isothiocyanates by the two procedures in satisfactory yields. Halogens, benzylic C–H bonds, methylthio, nitro, ester, alkenyl, electron-rich or -deficient (hetero)aryls, acetylenyl, and even phenolic and alcoholic hydroxyls are well tolerated. The one-pot procedure in water can also be used to realize the preparation of chiral isothiocyanates from chiral amines, and the modification of bioactive structures with free amino groups. In large-scale preparation, simple and practical purification procedures independent of column chromatography are developed.


 Please wait while we load your content...
Please wait while we load your content...