Metal-free, regioselective and stereoregular alternating copolymerization of monosubstituted epoxides and tricyclic anhydrides†
Abstract
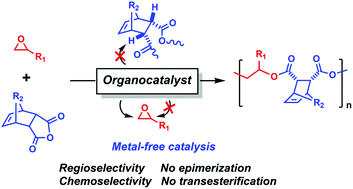
A metal-free, highly regioselective and stereoregular ring-opening alternating copolymerization (ROAC) of monosubstituted epoxides with tricyclic anhydrides remains a challenge in the advancement of polyester synthesis. Herein, we described an effective group of organic dual catalysts for the ROAC, exhibiting a high catalytic activity (the highest TOF = 330 h−1 at 110 °C), narrow polydispersity (PDI < 1.20) and excellent alternating selectivity (ester > 99%) in a controlled manner. The ROAC of a variety of monosubstituted epoxides and tricyclic anhydrides was carried out under mild conditions. Of importance is the fact that highly regioselective insertion of epoxides has been realized by simple and metal-free catalysts in the ROAC, where the highest regioselectivity is up to 98% for aliphatic epoxides and glycidyl ethers at 80 °C. Styrene oxide bearing electron-withdrawing phenyl also showed a good regioselectivity of 78%. Besides, the complete suppression of epimerization and transesterification was achieved even at high conversion for a variety of tricyclic anhydrides. Furthermore, block and gradient copolymers were synthesized by the sequential addition strategy and one-pot terpolymerization. Accordingly, a green, regioselective and stereoregular fabrication of functional polyesters was realized for the first time by a metal-free process.


 Please wait while we load your content...
Please wait while we load your content...