Copper-catalyzed pyrrole synthesis from 3,6-dihydro-1,2-oxazines†
Abstract
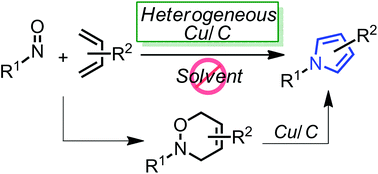
Highly-functionalized pyrroles could be effectively synthesized from 3,6-dihydro-1,2-oxazines using a heterogeneous copper on carbon (Cu/C) under neat heating conditions. Furthermore, the in situ formation of 3,6-dihydro-1,2-oxazines via the hetero Diels–Alder reaction between nitroso dienophiles and 1,3-dienes and the following Cu/C-catalyzed pyrrole synthesis also provided the corresponding pyrrole derivatives in a one-pot manner.


 Please wait while we load your content...
Please wait while we load your content...