Catalyst-free three-component synthesis of highly functionalized 2,3-dihydropyrroles†
Abstract
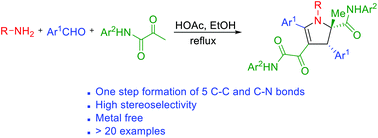
An efficient synthesis of fully substituted 2,3-dihydropyrroles has been achieved in one step through the three-component reaction of amines, aromatic aldehydes and α-ketoamides. This atom-economical and catalyst-free reaction is highly stereoselective and generates underexplored heterocycles in a single step. These compounds were examined in an enzymatic assay that led to the identification of potent α-glucosidase inhibitors, thereby demonstrating the utility of this novel methodology in medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...