Comment on “Zemplén transesterification: a name reaction that has misled us for 90 years” by B. Ren, M. Wang, J. Liu, J. Ge, X. Zhang and H. Dong, Green Chemistry, 2015, 17, 1390–1394†
Abstract
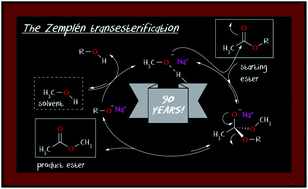
The recent title paper reports that the Zemplén transesterification can be accomplished equally well using catalytic amounts of NaOH in MeOH instead of the classical catalytic amounts of MeONa in dry MeOH, which led the authors to propose a modified mechanism for this transformation. Simple proton transfer considerations show that, in the above modified conditions, the original methoxy anion-catalysed mechanistic path still holds against the proposed hydroxy anion-catalysed one, when hydrogen bonding is taken into account.


 Please wait while we load your content...
Please wait while we load your content...