Inhibitory effects of catechins on β-carbolines in tea leaves and chemical model systems
Abstract
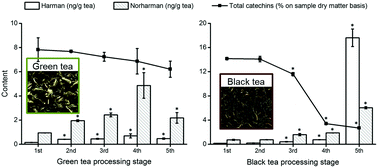
The profile of 18 heterocyclic amines from seven categories (including β-carbolines) in tea leaves during green and black tea processing procedures, as well as commercial tea products was screened by ultrahigh-performance liquid chromatography with tandem mass spectrometry. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ), and 2-amino-3,8-dimethyl-imidazo[4,5-f]quinoxaline (MeIQx) were detected in samples; however, the levels were too low for quantification. The β-carboline compounds harman and norharman were quantified in all the samples. During processing, both harman and norharman levels increased significantly (p < 0.01) with the highest levels present in tea leaves during the rolling stage of green tea processing and the drying stage of black tea processing. In commercial products, the highest levels of harman and norharman were found in black tea as 31.49 ± 3.21 and 59.68 ± 4.71 ng g−1 sample, respectively. In combination with the catechin levels of tea leaves and the results of chemical model systems, it was demonstrated that ECG, EGC, and EGCG could significantly (p < 0.01) inhibit the formation of harman and norharman by up to 45% and 52%, respectively.


 Please wait while we load your content...
Please wait while we load your content...