Retracted Article: The influence of nanoscale size on the adsorption–desorption of U(vi) on nano-Al oxides†
Abstract
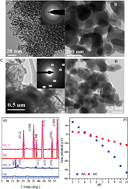
Understanding the effect of nanoscale size on the adsorption–desorption of U(VI) on nano-Al oxides is critical in predicting the transportation and fate of U(VI) in aquifers due to the ubiquitous presence of reactive nano-particles in subsurface sediments. In this study, the adsorption–desorption of U(VI) on nano-Al oxides (NA) and natural corundum (NC) was carried out under various environmental conditions by batch techniques. The characteristic results showed that surface reactive sites of NA (4280 μmol g−1) were approximately two orders of magnitude higher than that of NC (35 μmol L−1). The maximum adsorption capacities of NA and NC at pH 6.0 and 293 K were 188.68 and 58.48 mg g−1, respectively. Batch desorption demonstrated that NA presented multiple surface reactive sites, which was consistent with the results of surface complexation modeling. The results of XPS and EXAFS spectra showed that NA displayed strong chemical affinity for U(VI) compared to NC, indicating that the main interaction mechanism of U(VI) on NA was inner-sphere surface complexation, which was then incorporated into the internal structure of the second mineral phase. These findings imply that the high adsorption capacity and strong chemical affinity of U(VI) removal on NA were due to the effect of nanoscale size, which plays a significant role in immobilizing U(VI) in surface sediments.


 Please wait while we load your content...
Please wait while we load your content...