Continuous electrochemical heat engines†
Abstract
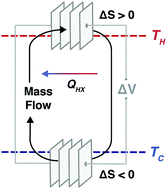
Given the large magnitude of energy in waste heat, its efficient conversion to electrical power offers a significant opportunity to lower greenhouse gas emissions. However, it has been difficult to optimize the performance of new direct energy conversion approaches because of the coupling between entropy change and thermal and electrical transport in continuously operating devices. With electrochemical cells driving flowing electrolytes in symmetric redox reactions at different temperatures, we demonstrate two continuous electrochemical heat engines that operate at 10–50 °C and at 500–900 °C, respectively. Simulations of kilowatt-scale systems using electrochemical cells stacked in series suggest efficiencies over 30% of the Carnot limit and areal power densities competitive with solid-state thermoelectrics at maximum power. Although entropy change, thermal transport and electrical transport are inherently coupled in solid-state thermoelectrics, they can be somewhat circumvented in electrochemical systems, thus offering new opportunities to engineer efficient energy conversion systems.


 Please wait while we load your content...
Please wait while we load your content...
