Efficient visible light-driven water oxidation and proton reduction by an ordered covalent triazine-based framework†
Abstract
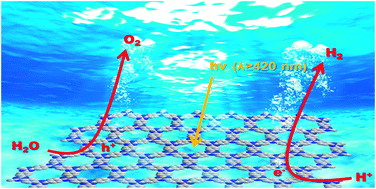
Water oxidation is a rate-determining step in solar driven H2 fuel synthesis and is technically challenging to promote. Despite decades of effort, only a few inorganic catalysts are effective and even fewer are effective under visible light. Recently, attention has been paid to synthetic semiconducting polymers, mainly on graphitic C3N4, with encouraging hydrogen evolution performance but lower activity for water oxidation. Here, a highly ordered covalent triazine-based framework, CTF-1 (C8N2H4), is synthesised by a very mild microwave-assisted polymerisation approach. It demonstrates extremely high activity for oxygen evolution under visible light irradiation, leading to an apparent quantum efficiency (AQE) of nearly 4% at 420 nm. Furthermore, the polymer can also efficiently evolve H2 from water. A high AQE of 6% at 420 nm for H2 production has also been achieved. The polymer holds great potential for overall water splitting. This exceptional performance is attributed to its well-defined and ordered structure, low carbonisation, and superior band positions.


 Please wait while we load your content...
Please wait while we load your content...