Effect of an alkyl substituent and spacer length in benzene-centered tripodal diglycolamides on the sequestration of minor actinides†
Abstract
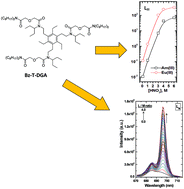
Three benzene-centered tripodal diglycolamide (Bz-T-DGA) ligands, where diglycolamide (DGA) moieties are tethered to the central benzene ring through a methylene spacer and having either a hydrogen atom (LI) or an isopentyl group (LII) attached to the N-atom, and DGA moieties attached via an ethylene spacer and having an isopentyl group attached to the N-atom (LIII), were studied for their complexation and extraction abilities towards trivalent actinides and lanthanides. The distribution ratio of Am(III) and Eu(III) with 1 mmol L−1 ligand in 5% iso-decanol/n-dodecane followed the order: LII > LIII > LI. The substitution of the H atom with the isopentyl group on the N-atom of the DGA moieties resulted in two orders of magnitude enhancement in the extraction ability of the ligand. On the other hand, increase in the spacer length between the benzene ring and the DGA moieties resulted in several fold reduction in the extraction ability of the ligand. Spectroscopic studies with Eu3+ ions in acetonitrile also confirmed the metal/ligand complex formation constant in the order: LII > LIII > LI. Luminescence decay lifetimes of Eu3+/ligand complexes confirmed the absence of water molecules and that all the primary coordination sites of the metal ion are occupied by the ligands.


 Please wait while we load your content...
Please wait while we load your content...