Mechanistic insights into the iridium catalysed hydrogenation of ethyl acetate to ethanol: a DFT study†
Abstract
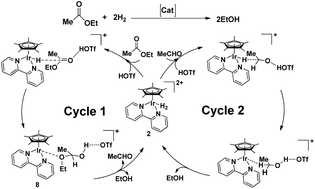
Density functional theory study of the hydrogenation of ethyl acetate catalysed by iridium complexes [Cp*Ir(bpy)OH2]2+ reveals a direct C–O bond cleavage mechanism with two cascade catalytic cycles for the hydrogenation of ethyl acetate to aldehyde and the hydrogenation of aldehyde to ethanol. Calculation results indicate that the rate-determining state in the whole catalytic reaction is the direct C–O bond cleavage for the formation of aldehyde and ethanol with a total free energy barrier of 25.5 kcal mol−1, which is 0.6 kcal mol−1 more favorable than the mechanism proposed by Goldberg and co-workers in their experimental study.


 Please wait while we load your content...
Please wait while we load your content...