The chameleonic reactivity of dilithio bis(alkylamido)cyclodiphosph(iii)azanes with chlorophosphines†
Abstract
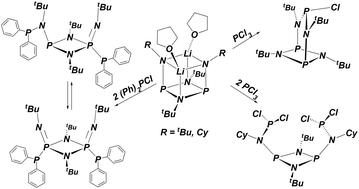
To synthesize a bis(diphosphinylamine) ligand for use in late-metal catalysis dilithio bis(tert-butylamido)cyclodiphosph(III)azane was treated with two equivalents of (Ph)2PCl. The chlorodiphenylphosphine attacked the dilithium salt kinetically at phosphorus, followed by a rearrangement to an unsymmetrical P, N-di-substituted product. Solid-state structures of both products were determined, and the activation energy for the rearrangement was measured. To gain further insight into the location of chlorophosphine attack (N versus P) on lithium diamides, dilithio bis(cyclohexylamido)cyclodiphosph(III)azane or dilithio bis(tert-butylamido)cyclodisilazane was treated with two equivalents of PCl3. In each case only the symmetrically N,N′-substituted bis(PCl2) product was obtained and characterized by X-ray analysis. The structures of these latter compounds are unusual, because in both cases a bicyclic compound with a bis(amido) chelated, central P–Cl moiety was expected based on precedent. The two proximal and juxtaposed PCl2 groups may provide a starting point for potentially novel reactivity studies.


 Please wait while we load your content...
Please wait while we load your content...