An air and moisture tolerant iminotrihydroquinoline-ruthenium(ii) catalyst for the transfer hydrogenation of ketones†
Abstract
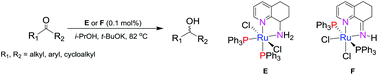
Reaction of 8-amino-5,6,7,8-tetrahydroquinoline with RuCl2(PPh3)3 at room temperature affords the ruthenium(II) chelate (8-NH2-C9H10N)RuCl2(PPh3)2 (E), in which the two triphenylphosphine ligands are disposed mutually cis. By contrast, when the reaction is performed at reflux ligand oxidation/dehydrogenation occurs along with cis–trans reorganization of the triphenylphosphines to form the 8-imino-5,6,7-trihydroquinoline-ruthenium(II) complex, (8-NH-C9H9N)RuCl2(PPh3)2 (F). Complex F can also be obtained in higher yield by heating a solution of E alone to reflux. Comparison of their molecular structures highlights the superior binding properties of the bidentate imine ligand in F over its amine-containing counterpart in E. Both complexes are highly effective in the transfer hydrogenation of a wide range of alkyl-, aryl- and cycloalkyl-containing ketones affording their corresponding secondary alcohols with loadings of as low as 0.1 mol%. Significantly, F can deliver excellent conversions even in bench quality 2-propanol in reaction vessels open to the air, whereas the catalytic efficiency of E is diminished by the presence of air but only operates efficiently under inert conditions.


 Please wait while we load your content...
Please wait while we load your content...