Nickel foam derived nitrogen doped nickel sulfide nanowires as an efficient electrocatalyst for the hydrogen evolution reaction†
Abstract
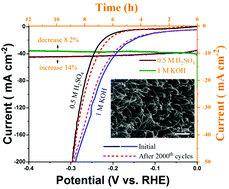
Ni3S2 has been validated as an effective electrocatalyst for the oxygen evolution reaction, attributable to its suitable electronic configuration. However, pure Ni3S2 towards the hydrogen evolution reaction (HER) exhibits relatively low catalytic activity. Herein, a one-step annealing strategy is demonstrated for the synthesis of Ni3S2 nanowires on nickel foam (NF) through N doping towards vigorous HER performance both in acid and alkaline solution. The 1D N-Ni3S2 nanowires, integrated onto a 3D NF electrode, show a high catalytic current density of 10 mA cm−2 at a low overpotential of 196 mV and a small Tafel slope of 63 mV dec−1 in an acid electrolyte. In an alkaline medium, the N-Ni3S2 NWs exhibit a low overpotential of 105 mV at the current density of 10 mA cm−2, which is lower than other reported Ni3S2-based HER catalysts. The superior HER catalytic performance is attributed to its unique structural features, both the morphology and electronic structure. Our work provides profound guidance for the design and optimization of electrocatalysts for an efficient HER.


 Please wait while we load your content...
Please wait while we load your content...