Access to a pair of ambiphilic phosphine–borane regioisomers by rhodium-catalyzed hydroboration†
Abstract
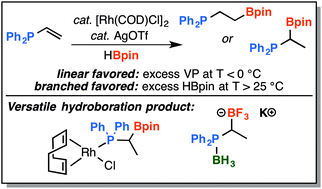
Lewis basic substrates, such as vinylphosphines and enamines, can be problematic for transition-metal catalysed hydrofunctionalization reactions due to their propensity to ligate and deactivate transition-metal catalysts as well as form direct Lewis adducts with reaction partners. While exploring rhodium-catalyzed hydroboration of diphenylvinylphosphine with pinacolborane, we found that a high degree of regiocontrol could be achieved without the need to diminish the Lewis basicity of the phosphine by oxidation or prior-protection. At slightly elevated temperature, a high yield of the previously unreported branched regioisomer, 1-pinacolatoborono-1-diphenylphosphinoethane, was achieved with regioselectivity greater than 10 : 1 using [Rh(COD)Cl]2 as the catalyst and AgOTf as a catalytic additive. Inversion of regioselectivity occurred at low temperature and high yield of the linear regioisomer was observed. Subsequent functionalization of the new branched phosphine–boronic ester and its coordination to rhodium were also investigated.


 Please wait while we load your content...
Please wait while we load your content...