Stable ruthenium olefin metathesis catalysts bearing symmetrical NHC ligands with primary and secondary N-alkyl groups†
Abstract
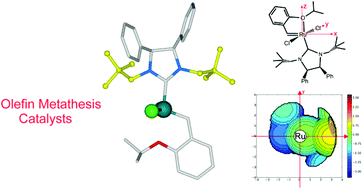
Four novel stable Hoveyda–Grubbs-type catalysts containing N,N′-dineopentyl- and N,N′-dicyclohexyl-substituted N-heterocyclic carbene (NHC) ligands with syn and anti phenyl groups on the ring backbone were synthesized and fully characterized. The catalytic potential of these complexes was investigated in metathesis reactions of both standard and renewable substrates. Compared to the Hoveyda–Grubbs second generation catalyst (HGII), all of the new catalysts showed high performances in most of the examined metathesis transformations. In particular, N,N′-dicyclohexyl catalysts gave improved results in the challenging ring-closing metathesis (RCM) reaction to form tetrasubstituted olefins, while catalysts with neopentyl N-groups were found to be more active and Z-selective in cross-metathesis (CM) reactions. Modest enantioselectivities in the asymmetric ring-closing metathesis (ARCM) of achiral trienes with different steric hindrance were observed in the presence of catalysts bearing chiral C2-symmetric NHC ligands.


 Please wait while we load your content...
Please wait while we load your content...