Electrodeposited-film electrodes derived from a precursor dinitrosyl iron complex for electrocatalytic water splitting†
Abstract
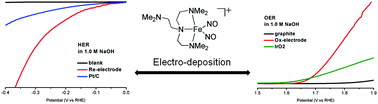
In artificial photosynthesis, water splitting plays an important role for the conversion and storage of renewable energy sources. Here, we report a study on the electrocatalytic properties of the electrodeposited-film electrodes derived from irreversible electro-reduction/-oxidation of a molecular dinitrosyl iron complex (DNIC) {Fe(NO)2}9 [(Me6tren)Fe(NO)2]+ (Me6tren = tris[2-(dimethylamino)ethyl]amine) for the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) in alkaline solution, individually. For HER, the overpotential and Tafel slope for the electrodeposited-film cathode are lower than those of the equiv.-weight Pt/C electrode. The electrodeposited-film anode for the OER is stable for 139 h. Integration of the electrodeposited-film cathode and anode into a single electrode-pair device for electrocatalytic water splitting exhibits an onset voltage of 1.77 V, achieving a geometrical current density of 10 mA cm−2.


 Please wait while we load your content...
Please wait while we load your content...