Novel antimony(iii) hydroxamic acid complexes as potential anti-leishmanial agents†
Abstract
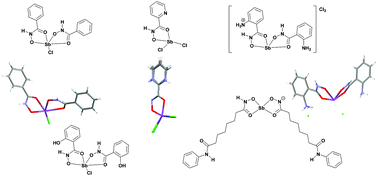
Reaction of benzohydroxamic acid (Bha), 2-pyridinehydroxamic acid (2-pyha), 2-amino-phenylhydroxamic acid (2-NH2-pha) and salicylhydroxamic acid (Sha) with SbCl3 in ethanol gave the corresponding novel hydroxamato Sb(III) complexes, [Sb(Bha-1H)2Cl] 1, [SbCl2(2-pyha-1H)] 2, [Sb(2-NH2-pha-1H)(2-NH3-pha-1H)]Cl23 and [SbCl(Sha-1H)2] 4. In all cases the hydroxamic acids coordinate to the Sb centres in the typical bidentate hydroxamato (O,O′) coordination mode, via the carbonyl oxygen and deprotonated hydroxyl group. Reaction of the histone deacetylase inhibitor (HDACi) suberoylanilidehydroxamic acid (SAHA) with Sb(OEt)3 gave the Sb(III) hydroxamato/hydroximato complex, [Sb(SAHA-1H)(SAHA-2H)] 6. All test complexes significantly inhibited the promastigote proliferation of Leishmania amazonensis and L. chagasi and induced substantial changes in the general morphology of the parasites, including reduction in size and loss of flagellum, when compared to the untreated promastigotes. A dose–response approach using the test complexes showed a decreased in plasma membrane permeability and the mitochondrial dehydrogenase activities of the Leishmania species. [Sb(Bha-1H)2Cl] exhibited the best activity and was superior to the Sb HDACi complex 6. Though 1 exhibited noteworthy anti-leishmanial activity, the selectivity indexes determined suggest that [Sb(2-NH2-pha-1H)(2-NH3-pha-1H)]Cl23 is the test complex that merits further investigation as a potential anti-leishmanial agent.


 Please wait while we load your content...
Please wait while we load your content...