Dinitrogen photoactivation: status quo and future perspectives
Abstract
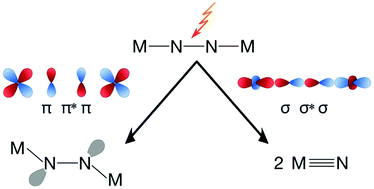
While the thermal activation of the dinitrogen molecule has been demonstrated in numerous examples with a variety of transition metals and ligand frameworks, the use of light to induce a weakening or splitting of the strong N–N bond is less well explored. Six complexes that bind N2 in a linear μ–η1:η1-end-on fashion between two transition metals are known to cleave dinitrogen after absorbing a photon and relaxing from an electronically excited state. This Perspective article reviews the molecular complexes known to be capable of dinitrogen photocleavage, and discusses mechanistic insights into the photoactivation process gained from experimental and computational studies. In an extension of previous hypotheses for pathways to dinitrogen photoactivation that would facilitate easy protonation of the μ-N atoms, a scheme is presented that may help to identify other complexes that could be capable of dinitrogen photoactivation.
- This article is part of the themed collections: 2018 Frontier and Perspective articles and New Talent: Europe


 Please wait while we load your content...
Please wait while we load your content...