The structural and electronic properties of 3,3′-azothiophene photo-switching systems†
Abstract
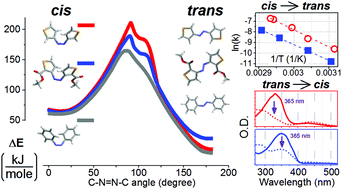
A diversity of photo-switching structural elements opens up new opportunities in the engineering of light driven reshaping of matter, in catalysis on-click including photodynamic cancer therapy, in light sensitive transport control and in data storage. With the assistance of quantum calculations we explore the photo-physical properties of novel 3,3′-azothiophene molecular systems, the synthesis of which we reported recently. In the considered azothiophenes, upon exposure to ultraviolet and visible radiation, we observed effective anti(trans) to syn(cis) and syn(cis) to anti(trans) isomerization of the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– moiety, respectively. In contrast to azobenzene based photo-switchable molecular systems, the syn(cis) to anti(trans) isomerization in the azothiophenes studied does not take place at 22 °C in the dark. Temperature dependent experiments and theoretical studies suggest a slightly higher barrier for such processes than for azobenzene, which we attribute to the specific structural and electronic properties of the thiophene ring and the nature of the side groups. We discuss the potential of the observed properties in the development of novel molecular photo-switching machinery to promote biocatalytic applications at interfaces.
N– moiety, respectively. In contrast to azobenzene based photo-switchable molecular systems, the syn(cis) to anti(trans) isomerization in the azothiophenes studied does not take place at 22 °C in the dark. Temperature dependent experiments and theoretical studies suggest a slightly higher barrier for such processes than for azobenzene, which we attribute to the specific structural and electronic properties of the thiophene ring and the nature of the side groups. We discuss the potential of the observed properties in the development of novel molecular photo-switching machinery to promote biocatalytic applications at interfaces.


 Please wait while we load your content...
Please wait while we load your content...
