Li-decorated carbon ene–yne as a potential high-capacity hydrogen storage medium†
Abstract
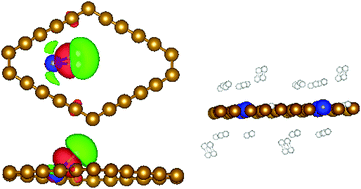
Based on comprehensive first-principles calculations, we predict that Li-decorated carbon ene–yne (CEY) can serve as a reversible and high density hydrogen storage medium. The adsorption structures, charge transfer, electronic properties and energy storage characteristics of Li-decorated CEY are investigated. It is established that Li can bind strongly to sp and sp2 hybridized CEY without the formation of Li clusters. The strong interaction between Li and CEY is attributed to the sp binding C px/py states which hybridize heavily with the Li 2s/2p states. After Li decoration, CEY undergoes a transition from a semiconductor to a metal. Each Li atom anchors seven H2 molecules with the average adsorption energy falling in a suitable range (−0.2 to −0.4 eV per H2). The adsorption mechanism of H2 molecules has also been discussed. Two Li atom decorated CEY can obtain up to a 9.96 wt% hydrogen storage capacity.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...