Li+-Induced fluorescent metallogel: a case of ESIPT-CHEF and ICT phenomenon†
Abstract
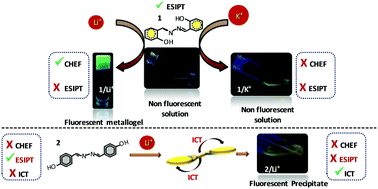
A fluorescent metallogel (1% w/v) has been synthesized from non-fluorescent ingredients viz. the smallest possible low molecular weight aromatic symmetrical ligand H2SA (1) and LiOH in a chloroform and methanol mixture. The chelation of Li+ is not only responsible for the inhibition of excited state intramolecular proton transfer (ESIPT) or the origin of fluorescence through chelation enhanced fluorescence (CHEF) in 1, but also for aggregation leading to gelation. The metallogel obtained from 1/Li+ reveals a fibrous morphology while 1 with other, bigger size, alkali metal ions like Na+/K+/Cs+ demonstrates the growth of crystals with different shapes. The effect of the size of the alkali metal ion over gel formation is well explored by FTIR, UV-vis, fluorescence, average lifetime measurements, SEM and PXRD. The metallogel shows multi-stimuli responsive behaviour towards thermal and mechanical stress as well as reswelling properties. The regioisomer H2PBA (2) also shows emission upon treatment with LiOH due to the presence of intramolecular charge transfer (ICT), this is well established by various experiments. The mechanism of gel formation is well established by FTIR, 1H NMR, UV-vis, fluorescence, lifetime measurements, SEM and single crystal and powder XRD instrumental techniques. The involvement of various phenomena in gel formation has been further supported by other synthesized model compounds viz. H2MBA (3), PMO (4), H2SEA (5) and H2SPA (6). True gel phase material is proved by detailed rheological experiments.


 Please wait while we load your content...
Please wait while we load your content...