Modeling of solid–liquid interfaces using scaled charges: rutile (110) surfaces†
Abstract
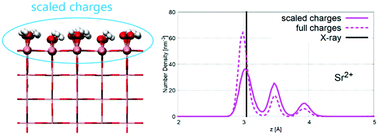
Electronic continuum correction (ECC) has been proven to bring significant improvement in the modeling of interactions of ions (especially multivalent) in aqueous solutions. We present a generalization and the first application of this approach to modeling solid–liquid interfaces, which are omnipresent in physical chemistry, geochemistry, and biophysics. Scaling charges of the top layer of surface atoms makes the existing solid models compatible with the ECC models of ions and molecules, allowing the use of modified force fields for a more accurate investigation of interactions of various metal and metal-oxide surfaces with aqueous solutions, including complex biomolecules and multivalent ions. We have reparametrized rutile (110) models with different surface charge densities (from 0 to −0.416 C m−2) and adopted/developed scaled charge force fields for ions, namely Na+, Rb+, Sr2+, and Cl−. A good agreement of the obtained molecular dynamics (MD) data with X-ray experiments and previously reported MD results was observed, but changes in the occupancy of various adsorption sites were observed and discussed in detail.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...