Theoretical investigations on hydrogen peroxide decomposition in aquo†
Abstract
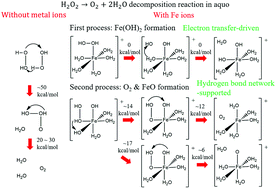
Hydrogen peroxide (H2O2) decomposition mechanisms in the absence and presence of iron ions in aqueous solution, which contain no OH radical formation, are theoretically determined. Calculating the oxygen–oxygen bond dissociation energies of H2O2, we confirmed that OH radical formation requires spin-forbidden transitions. Instead, we tested an H2O2 dimer-based decomposition mechanism and found that this mechanism provides reasonable barrier heights of 52–62 kcal mol−1, which are close to the experimental activation energy. We next calculated the oxygen–oxygen bond dissociation of H2O2 coordinating to the iron ion hydration complex in order to explore H2O2 decomposition in the presence of iron ions. Surprisingly, we found that a monovalent iron ion complex provides no reaction barrier to dissociate H2O2, in contrast to the ferrous (Fe2+) and ferric (Fe3+) ion complexes with accompanying very high barriers. Following this result, we determined the subsequent oxygen formation mechanism of the monovalent iron ion complex and found that this mechanism needs a hydrogen bond network around H2O2 to proceed at room temperature. We, therefore, conclude that H2O2 decomposition in the presence of iron ions is driven by electron transfer to the iron ion hydration complex and proceeds by hydrogen transfers in the hydrogen bond network around H2O2.


 Please wait while we load your content...
Please wait while we load your content...