A physiochemical processing kinetics model for the vapor phase infiltration of polymers: measuring the energetics of precursor-polymer sorption, diffusion, and reaction†
Abstract
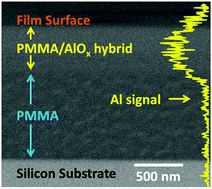
Vapor phase infiltration (VPI) is a new approach for transforming polymers into organic–inorganic hybrid materials with unique properties. Here, we combine experimental measurements with phenomenological theory to develop a universal strategy for measuring, modeling, and predicting the processing kinetics of VPI. We apply our approach to the well-studied VPI system of trimethylaluminum (TMA) infiltrating poly(methyl methacrylate) (PMMA) because the system undergoes both precursor-polymer diffusion and reaction. By experimentally measuring aluminum concentration profiles as a function of film depth with secondary ion mass spectrometry (SIMS) and film swelling with ellipsometry, we have extracted equilibrium solubility and effective diffusivity as a function of process temperature. Fitting these values to appropriate Van’t Hoff and Arrhenius relationships, we can then extract enthalpies for precursor sorption and diffusion. We observe an abrupt mechanistic change in both the sorption and diffusion processes around 95 °C, where greater chain mobility at higher processing temperatures lead to greater reactivity between TMA and PMMA. With new understanding of this VPI process, we demonstrate precise control of inorganic infiltration depth and loading fraction into PMMA.


 Please wait while we load your content...
Please wait while we load your content...
