Description of colloidal particles aggregation in the presence of Hofmeister effects: on the relationship between ion adsorption energy and particle aggregation activation energy†
Abstract
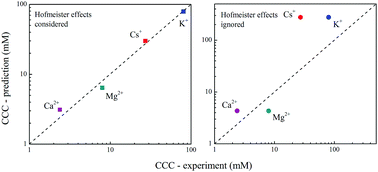
Particle aggregation is acutely affected by Hofmeister effects. Results for aggregation behavior in the presence of Hofmeister effects predicted by the classic DLVO model were not satisfactory. In this study, description of colloidal clay particles aggregation in the presence of Hofmeister effects based on a theoretical relationship between ion adsorption energy and aggregation activation energy was established. Moreover, the validity of the suggested theory was confirmed with the published experimental data on montmorillonite particles aggregation in solutions of LiNO3, KNO3, CsNO3, Mg(NO3)2 and Ca(NO3)2. In the presence of Hofmeister effects, the differences in adsorption ability of the involved five cations were quantitatively characterized by defining an additional Hofmeister energy. We found that the additional Hofmeister energy for Li+, K+, Cs+, Mg2+ and Ca2+ on montmorillonite surface were 0.063, 0.942, 1.864, 0.850 and 2.010-times larger, respectively, than the classic Coulomb interaction energy. Taking these additional Hofmeister energies into account, CCC values for the presence of different cations were theoretically calculated by the suggested theory, and the predicted CCC values matched well with the experimental results. The theoretically predicted CCC values in montmorillonite aggregation for KNO3, CsNO3, Mg(NO3)2 and Ca(NO3)2 were 78.8, 29.9, 6.48, and 3.12 mM, respectively, and the corresponding measured CCC values were 80.3, 27.2, 7.99, and 2.38 mM. Our findings are helpful for further understanding the interactions of nanoparticles with cations and quantitatively answer how ion–surface interactions affect particle interaction processes.


 Please wait while we load your content...
Please wait while we load your content...