Effects of hydrogen bonding on the gas-phase reactivity of didehydroisoquinolinium cation isomers†
Abstract
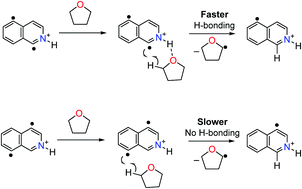
Two previously unreported isomeric biradicals with a 1,4-radical topology, the 1,5-didehydroisoquinolinium cation and the 4,8-didehydroisoquinolinium cation, and an additional, previously reported isomer, the 4,5-didehydroisoquinolinium cation, were studied to examine the importance of the exact location of the radical sites on their reactivities in the gas phase. The experimental results suggest that hydrogen bonding in the transition state enhances the reactivity of the 1,5-didehydroisoquinolinium cation towards tetrahydrofuran but not towards allyl iodide, dimethyl disulfide or tert-butyl isocyanide. The observation of no such enhancement of reactivity towards tetrahydrofuran for the 4,8-didehydroisoquinolinium and 4,5-didehydroisoquinolinium cations supports this hypothesis as these two biradicals are not able to engage in hydrogen bonding in their transition states for hydrogen atom abstraction from tetrahydrofuran. Quantum chemical transition state calculations indicate that abstraction of a hydrogen atom from tetrahydrofuran by the 1,5-didehydroisoquinolinium cation occurs at the C-1 radical site and that the transition state is stabilized by hydrogen bonding.


 Please wait while we load your content...
Please wait while we load your content...
