Experimental study and computational modelling of cruzain cysteine protease inhibition by dipeptidyl nitriles
Abstract
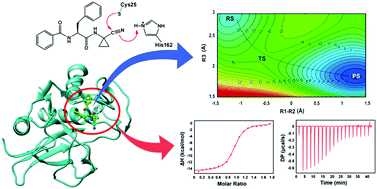
Chagas disease affects millions of people in Latin America. This disease is caused by the protozoan parasite Trypanossoma cruzi. The cysteine protease cruzain is a key enzyme for the survival and propagation of this parasite lifecycle. Nitrile-based inhibitors are efficient inhibitors of cruzain that bind by forming a covalent bond with this enzyme. Here, three nitrile-based inhibitors dubbed Neq0409, Neq0410 and Neq0570 were synthesized, and the thermodynamic profile of the bimolecular interaction with cruzain was determined using isothermal titration calorimetry (ITC). The result suggests the inhibition process is enthalpy driven, with a detrimental contribution of entropy. In addition, we have used hybrid Quantum Mechanical/Molecular Mechanical (QM/MM) and Molecular Dynamics (MD) simulations to investigate the reaction mechanism of reversible covalent modification of cruzain by Neq0409, Neq0410 and Neq0570. The computed free energy profile shows that the nucleophilic attack of Cys25 on the carbon C1 of inhibitiors and the proton transfer from His162 to N1 of the dipeptidyl nitrile inhibitor take place in a single step. The calculated free energy of the inhibiton reaction is in agreement with covalent experimental binding. Altogether, the results reported here suggests that nitrile-based inhibitors are good candidates for the development of reversible covalent inhibitors of cruzain and other cysteine proteases.


 Please wait while we load your content...
Please wait while we load your content...