Structural and bonding properties of Cu3O3− and Cu3O4− clusters: anion photoelectron spectroscopy and density functional calculations†
Abstract
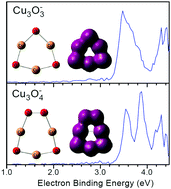
The structural and electronic properties of Cu3O3− and Cu3O4− were investigated using mass-selected anion photoelectron spectroscopy in combination with density functional theoretical calculations. The vertical detachment energies of Cu3O3− and Cu3O4− were measured to be 3.48 ± 0.08 and 3.54 ± 0.08 eV, respectively. Their geometrical structures were determined by comparison of the theoretical calculations with the experimental results. The most stable structure of Cu3O3− can be characterized as a C3v symmetric six-membered ring structure with alternating Cu–O bonds, in which the plane of the three O atoms is slightly above that of the three Cu atoms. The most stable structure of Cu3O4− can be viewed as a Cs symmetric seven-membered ring with a peroxo unit. The bond order and molecular orbital analyses indicate that the Cu–Cu interactions in Cu3O3− and Cu3O4− are weak. The calculated NICS(0) and NICS(1) values of Cu3O3− are −25.0 ppm and −19.2 ppm, respectively, and those of Cu3O4− are −18.6 ppm and −10.5 ppm, respectively, indicating that they both are significantly aromatic.


 Please wait while we load your content...
Please wait while we load your content...