Aggregation behavior of dihexadecylviologen bistriflimide ionic liquid crystal in different solvents: influence of polarity and concentration
Abstract
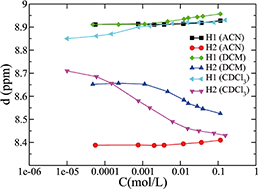
Solutions of 1,1′-dihexadecyl-4,4′-bipyridinium di[bis(trifluoromethanesulfonyl)imide] salt, also known as dihexadecylviologen bistriflimide, in deuterated acetonitrile (ACN), dichloromethane (DCM) and chloroform (CDCl3), respectively, were investigated by the combination of 1H and DOSY NMR spectroscopy, DFT calculations and MD simulation to understand the influence of solvent polarity and solute concentration (10−5–10−1 M) on its aggregation behavior. We found that the polar solvent acetonitrile (ACN) does not favor ion aggregation and cluster formation. In the whole range of concentrations investigated, the system appears to be dominated by neutral ion pairs composed of one cation and two anions, possibly in fast equilibrium (on the NMR time scale) with small or slightly larger aggregates. The diffusion coefficient of the cationic species is only weakly affected by concentration. In contrast, the low-polar solvents of chloroform (CDCl3) and dichloromethane (DCM) strongly favor cluster formation above a certain concentration and the viologen diffusion coefficient in CDCl3 is much smaller and more strongly dependent on concentration than that in ACN. The information obtained from the MD simulations suggests that the aggregates have a structure similar to the isotropic liquid phase of the viologen-based ionic liquids and ionic liquid crystals. The lifetimes of such large clusters appear to be relatively long, beyond the time scale of tens of nanoseconds. Moreover, the results from the aromatic proton NMR resonances provide some insights on the dielectric constants inside the viologen aggregates.


 Please wait while we load your content...
Please wait while we load your content...