Unveiling the complex vibronic structure of the canonical adenine cation†
Abstract
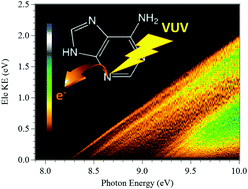
Adenine, a DNA base, exists as several tautomers and isomers that are closely lying in energy and that may form a mixture upon vaporization of solid adenine. Indeed, it is challenging to bring adenine into the gas phase, especially as a unique tautomer. The experimental conditions were tuned to prepare a jet-cooled canonical adenine (9H-adenine). This isolated DNA base was ionized by single VUV photons from a synchrotron beamline and the corresponding slow photoelectron spectrum was compared to ab initio computations of the neutral and ionic species. We report the vibronic structure of the X+ 2A′′ (D0), A+ 2A′ (D1) and B+ 2A′′ (D2) electronic states of the 9H adenine cation, from the adiabatic ionization energy (AIE) up to AIE + 1.8 eV. Accurate AIEs are derived for the 9H-adenine (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 1A′) + hν → 9H-adenine+ (X+ 2A′′, A+ 2A′, B+ 2A′′) + e− transitions. Close to the AIE, we fully assign the rich vibronic structure solely to the 9H-adenine (X 1A′) + hν → 9H-adenine+ (X+ 2A′′) transition. Importantly, we show that the lowest cationic electronic states of canonical adenine are coupled vibronically. The present findings are important for understanding the effects of ionizing radiation and the charge distribution on this elementary building block of life, at ultrafast, short, and long timescales.
1A′) + hν → 9H-adenine+ (X+ 2A′′, A+ 2A′, B+ 2A′′) + e− transitions. Close to the AIE, we fully assign the rich vibronic structure solely to the 9H-adenine (X 1A′) + hν → 9H-adenine+ (X+ 2A′′) transition. Importantly, we show that the lowest cationic electronic states of canonical adenine are coupled vibronically. The present findings are important for understanding the effects of ionizing radiation and the charge distribution on this elementary building block of life, at ultrafast, short, and long timescales.


 Please wait while we load your content...
Please wait while we load your content...