Electron localization in niobium doped CaMnO3 due to the energy difference of electronic states of Mn and Nb†
Abstract
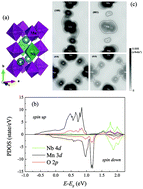
The electron localization in Nb-doped CaMnO3 is analyzed in terms of the space and energy distribution of electronic states employing first-principles calculations. The energy difference of Mn 3d states and Nb 4d states makes NbO6 octahedra impede electrical conduction, so the random distribution of Nb in lattices leads to the localization of electrons near the bottom of the conduction bands. Therefore, although more carriers are introduced when Nb-doping content increases, both the electrical conductivity and absolute thermopower decrease in Nb heavy doped CaMnO3. The calculated transport properties agree well with the experimental data, supporting the analysis of localization.


 Please wait while we load your content...
Please wait while we load your content...