Deciphering the chemoselectivity of nickel-dependent quercetin 2,4-dioxygenase†
Abstract
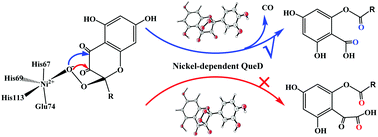
The reaction mechanism and chemoselectivity of nickel-dependent quercetin 2,4-dioxygenase (2,4-QueD) have been investigated using the QM/MM approach. The protonation state of the Glu74 residue, a first-shell ligand of Ni, has been considered to be either neutral or deprotonated. QM/MM calculations predict that Glu74 must be deprotonated to rationalize the chemoselectivity and steer the 2,4-dioxygenolytic cleavage of quercetin, which harvests the experimentally-observed product, 2-protocatechuoylphloroglucinol carboxylic acid, coupled with the release of carbon monoxide. If the enzyme has a neutral Glu74 residue, the undesired 2,3-dioxygenolytic cleavage of quercetin becomes the dominant pathway, leading to the formation of α-keto acid. The calculations suggest that the reaction takes place via three major steps: (1) attack of the superoxide on the C2 of the substrate pyrone ring to generate a NiII–peroxide intermediate; (2) formation of the second C–O bond between C4 and the peroxide to produce a peroxide bridge; (3) simultaneous cleavage of the C2–C3, C3–C4, and O1–O2 bonds with the formation of 2-protocatechuoylphloroglucinol carboxylic acid and carbon monoxide. The third step was found to be rate-limiting, with a barrier of 17.4 kcal mol−1, which is in very good agreement with the experimental kinetic data. For the second C–O bond formation, an alternative pathway is that the peroxide attacks the C3 of the substrate pyrone ring, leading to the formation of a four-membered ring intermediate, which then undergoes concerted C2–C3 and O1–O2 bond cleavages to produce an α-keto acid. This pathway is associated with a barrier of 30.6 kcal mol−1, which is much higher than the major pathway. When Glu74 is protonated, the 2,3-dioxygenolytic pathway, however, has a lower barrier (21.8 kcal mol−1) than the 2,4-dioxygenolytic pathway.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...