Probing the structural, electronic and magnetic properties of AgnSc (n = 1–16) clusters
Abstract
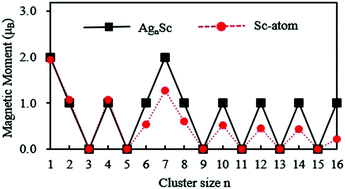
The structural, electronic and magnetic properties of AgnSc (n = 1–16) clusters have been studied on the basis of density functional theory and the CALYPSO structure prediction method. The optimized geometry exhibits that the growth process of Sc-doped silver clusters have a periodic structural change. The Ag atom grows around a basically invariant cluster core in each growth cycle. The Sc atom has a tendency to occupy the most highly coordinated position in the ground state. The infrared spectra, Raman spectra and photoelectron spectra of AgnSc clusters are forecasted and can be used to identify the structures of these clusters from experiments. The global maxima of the dissociation energy, the averaged binding energy and the gap of the energy level occur at n = 15 for the most stable AgnSc clusters, implying that the Ag15Sc can be perceived as a superatom. The magnetism analysis indicates that the magnetic moment of the Sc atom in AgnSc clusters decreases with the increase of the cluster. The change of the magnetic moment is proportional to the charge transfer between the Sc and Ag atoms.


 Please wait while we load your content...
Please wait while we load your content...