Improvements in the hydrogen storage properties of the Mg(NH2)2–LiH composite by KOH addition†
Abstract
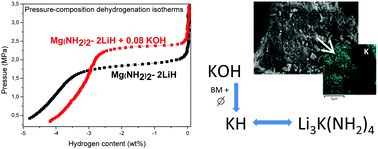
Potassium-containing compounds, such as KH, KOH, KNH2 and different potassium halides, have shown positive effects on the dehydrogenation properties of the Li–Mg–N–H system. However, it is still discussed whether the K-compounds modify the thermodynamics of the system or if they have only a catalytic effect. In this work the impact of the addition of two K-containing compounds (0.08 mol% of KCl and KOH) on the hydrogen storage performance of the Mg(NH2)2–LiH composite was studied. The KOH incorporation reduced the dehydrogenation temperature from 197 °C to 154 °C, beginning the process at low temperature (∼70 °C). The doped sample was able to reversibly absorb and desorb 4.6 wt% of hydrogen with improved kinetics; dehydrogenation rates were increased four times, whereas absorptions required 20% less time to be completed in comparison to the pristine material. The thermodynamic destabilization of the Mg(NH2)2–2LiH composite by the addition of a small amount of KOH was demonstrated by an increment of 30% in the dehydrogenation equilibrium pressure. According to detailed structural investigations, the KH formed by the KOH decomposition through milling and thermal treatment, can replace LiH and react with Mg(NH2)2 to produce a mixed potassium–lithium amide (Li3K(NH2)4). The KH role is not limited to catalysis, but rather it is responsible for the thermodynamic destabilization of the Mg(NH2)2–LiH composite and it is actively involved in the dehydrogenation process.


 Please wait while we load your content...
Please wait while we load your content...