DFT insight into the effect of potassium on the adsorption, activation and dissociation of CO2 over Fe-based catalysts†
Abstract
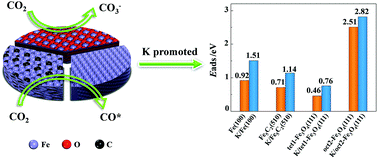
Catalytic conversion of CO2 including hydrogenation has attracted great attention as a method for chemical fixation of CO2 in combination with other techniques such as CO2 capture and storage. Potassium is a well-known promotor for many industrial catalytic processes such as in Fischer–Tropsch synthesis. In this work, we performed density functional theory (DFT) calculations to investigate the effect of potassium on the adsorption, activation, and dissociation of CO2 over Fe(100), Fe5C2(510) and Fe3O4(111) surfaces. The function of K was analyzed in terms of electronic interactions between co-adsorbed CO2 and K-surfaces which showed conspicuous promotion in the presence of K of the adsorption and activation of CO2. The adsorption strength of CO2 on these surfaces ranks as oct2-Fe3O4(111) > Fe(100) > Fe5C2(510). Generally, we observed a direct proportional correlation between the adsorption strength and the charges on the adsorbates. Adding K on the catalyst surface also reduces the kinetic barrier for CO2 dissociation. CO2 dissociation is more facile to occur on Fe(100) and Fe5C2(510) in the presence of K whereas the Fe3O4(111) surfaces impede CO2 dissociation regardless of the existence of K. Instead, a stable CO3− species is formed upon CO2 adsorption on Fe3O4(111) which will be directly hydrogenated when sufficient H* are available on the surface. Our results highlight the origin of the promotion effect of potassium and provide insight for the future design of K-promoted Fe-based catalysts for CO2 hydrogenation.


 Please wait while we load your content...
Please wait while we load your content...