Use multiscale simulation to explore the effects of the homodimerizations between different conformation states on the activation and allosteric pathway for the μ-opioid receptor†
Abstract
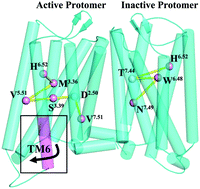
Recently, oligomers of G-protein coupled receptors (GPCRs) have been an important topic in the GPCR fields. However, knowledge about their structures and activation mechanisms is very limited due to the absence of crystal structures reported. In this work, we used multiscale simulations to study the effects of homodimerization between different conformation states on their activation, dynamic behaviors, and allosteric communication pathways for μ-OR. The results indicated that the dimerization of one inactive monomer with either one inactive monomer or one active one could enhance its constitutive activation. However, the conformation state of the other protomer (e.g., active or inactive) can influence the activated extent. The dimerization between the two inactive protomers leads to a negative cooperativity for their activation, which should contribute to the asymmetric activation of GPCR dimers observed in some experiments. On the other hand, for the active monomer, its dimerization with one inactive receptor could alleviate its deactivation, whereby negative and positive cooperativities can be observed between the two subunits of the dimer, depending on the different regions. Observations from protein structure network (PSN) analysis indicated that the dimerization of one inactive monomer with one active one would cause a significant drop in the number of main pathways from the ligand binding pocket to the G-protein coupled region for the inactive protomer, while the impact is minor for the active protomer. But, for the active monomer or the inactive one, its dimerization with one inactive monomer would significantly change the types of residues participating in the pathway with the highest frequency.


 Please wait while we load your content...
Please wait while we load your content...