Theoretical investigation of the strengthening mechanism and precipitation evolution in high strength Al–Zn–Mg alloys†
Abstract
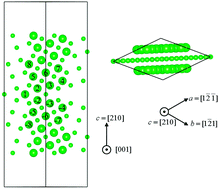
Density-functional theory calculations have been performed to systematically investigate the behaviors of solute atoms in 7000 series Al–Zn–Mg based alloys. It is found that solute atoms Mg and Zn are likely to segregate to the Σ5(210)[001] tilt Al GB. The bonding environment and interface cohesion will be affected to different degrees. Also, for GPI(100) our calculations indicate that a Zn/Mg/Zn sandwich configuration in the Al matrix (100) planes is energetically favorable. However, for GPII(111) the disordered structure turns out to be the most stable one. It mainly results from strong 3d–3s hybridization interactions between Zn and Mg atoms. Furthermore, the properties of the metastable phase η′ and the equilibrium phase η have also been addressed. The present study provides valuable insight for developing Al alloys with superior performance.


 Please wait while we load your content...
Please wait while we load your content...