A thermodynamically consistent phase diagram of a trimorphic pharmaceutical, l-tyrosine ethyl ester, based on limited experimental data†
Abstract
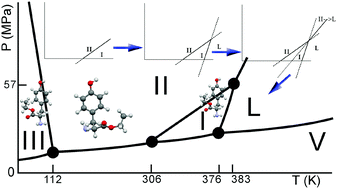
Crystalline polymorphs possess different physical properties, and phase changes between those polymorphs may affect the properties of engineered materials such as drugs. This is very well illustrated by the large effort that is put into the capability to predict phase behaviour of pharmaceuticals to avoid the unexpected appearance of different crystal forms. Much progress has been made, but one of the remaining challenges is (the accuracy in) the prediction of phase behaviour as a function of temperature. Obviously, predictions should at a certain point be verified against experimental data; however, it may not always be easy to elucidate the phase behaviour of a given compound experimentally, because thermodynamically and kinetically controlled phenomena occur in a convoluted fashion in experimental data. The present paper discusses the trimorphism of L-tyrosine ethyl ester as an example case of how experimental data in combination with the thermodynamic tenets lead to a consistent phase diagram, which can be used as the basis for pharmaceutical formulations and for comparison with polymorph predictions by computer. The positions of the two-phase equilibria I–II, I–III, and I–L have been obtained experimentally. Using the Clapeyron equation and the alternation rule, it has been shown how the positions of the other equilibria II–L, III–L, and II–III can be deduced in combination with the stability rankings of the phases and the phase equilibria. The experimental data have been obtained by synchrotron X-ray diffraction, Raman spectroscopy, and thermal analysis as a function of pressure and temperature. Furthermore, laboratory X-ray diffraction as a function of temperature and differential scanning calorimetry have been used. At room temperature, form II is the most stable phase, which remains stable with increasing pressure, as it possesses the smallest specific volume. Form I becomes stable above 33 °C (306 K), but with increasing pressure it turns into form III. On thermodynamic grounds, form III is expected to have a stable domain at very low temperatures.


 Please wait while we load your content...
Please wait while we load your content...