Nitrogen electroreduction and hydrogen evolution on cubic molybdenum carbide: a density functional study†
Abstract
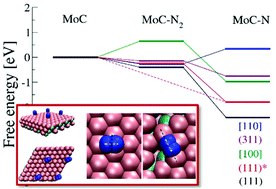
We report herein a density functional theory study of the nitrogen electroreduction and hydrogen evolution reactions on cubic molybdenum carbide (MoC) in order to investigate the viability of using this material as an electro-catalyst for ammonia synthesis. Free energy diagrams for associative and dissociative Heyrovsky mechanisms showed that nitrogen reduction on cubic MoC(111) can proceed via an associative mechanism and that small negative potentials of −0.3 V vs. standard hydrogen electrode can onset the reduction of nitrogen to ammonia. Kinetic volcano plots for hydrogen evolution showed that the MoC[110] surface is expected to have a high rate for the hydrogen evolution reaction, which could compete with the reduction of nitrogen on cubic MoC. The comparison between the adsorption energies of H-adatoms and N-adatoms also shows that at low potentials adsorption of hydrogen atoms competes with nitrogen adsorption on all the MoC surfaces except the MoC(111) surface. The hydrogen evolution and accumulation of H-adatoms can be mitigated by introducing carbon vacancies i.e. increasing the ratio of metal to carbon atoms, which will significantly increase the affinity of the catalytic surface for both nitrogen molecules and N-adatoms.


 Please wait while we load your content...
Please wait while we load your content...
