Reversible room temperature ammonia gas absorption in pore water of microporous silica–alumina for sensing applications
Abstract
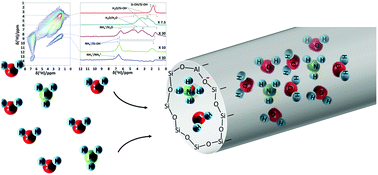
Microporous silica and silica–alumina powders exhibit a reversible uptake and release of ammonia gas from water vapor containing gas mixtures at ambient temperature, with capacities of 0.9 and 2.0 mmol g−1 in the presence of 100 ppm and 1000 ppm NH3, respectively. The ammonia trapping mechanism was revealed using a combination of direct excitation 1H MAS, 1H–1H EXSY and 1H DQ–SQ NMR spectroscopy, indicating that the major part of the captured ammonia is blended in the hydrogen bonded water network in the pores of the adsorbent. A small fraction is irreversibly bound as result of protonation and chemisorption. While common ammonia adsorbents need thermal regeneration, microporous silica–alumina can be regenerated by sweeping with dry gas at ambient temperature, desorbing the physisorbed fraction together with occluded water. As carbon dioxide does not interfere with the ammonia absorption process, this reversible absorption process of ammonia gas at ambient temperature is particularly attractive for sensor applications.


 Please wait while we load your content...
Please wait while we load your content...