Thermal reduction of ceria nanostructures on rhodium(111) and re-oxidation by CO2†
Abstract
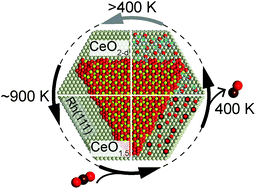
The thermal reduction of cerium oxide nanostructures deposited on a rhodium(111) single crystal surface and the re-oxidation of the structures by exposure to CO2 were investigated. Two samples are compared: a rhodium surface covered to ≈60% by one to two O–Ce–O trilayer high islands and a surface covered to ≈65% by islands of four O–Ce–O trilayer thickness. Two main results stand out: (1) the thin islands reduce at a lower temperature (870–890 K) and very close to Ce2O3, while the thicker islands need higher temperature for reduction and only reduce to about CeO1.63 at a maximum temperature of 920 K. (2) Ceria is re-oxidized by CO2. The rhodium surface promotes the re-oxidation by splitting the CO2 and thus providing atomic oxygen. The process shows a clear temperature dependence. The maximum oxidation state of the oxide reached by re-oxidation with CO2 differs for the two samples, showing that the thinner structures require a higher temperature for re-oxidation with CO2. Adsorbed carbon species, potentially blocking reactive sites, desorb from both samples at the same temperature and cannot be the sole origin for the observed differences. Instead, an intrinsic property of the differently sized CeOx islands must be at the origin of the observed temperature dependence of the re-oxidation by CO2.


 Please wait while we load your content...
Please wait while we load your content...