Interaction between amino-functionalized inorganic nanoshells and acid-autocatalytic reactions†
Abstract
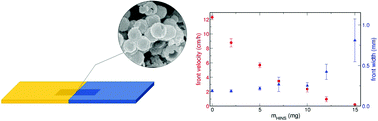
Amino-functionalized inorganic silica nanoshells with a diameter of 511 ± 57 nm are efficiently used as hydrogen ion binders with a base dissociation constant of (1.2 ± 0.1) × 10−4. The hydrogen removal has been shown to produce reaction–diffusion fronts of constant propagation velocity in the autocatalytic chlorite–tetrathionate reaction when it is run in thin planar slices of nanoshell-containing agarose gel to exclude all convection related effects. By controlling the exact amount of amino-functionalized hollow nanospheres in the gel matrix it is possible to finely tune the propagation velocity of the chemical front in the 0.1–10 cm h−1 range. Remarkably, this can be achieved with very low amino-functionalized hollow inorganic nanosphere loadings between 0.1–0.01 (m V−1)%. The front width has also been determined experimentally, which increases by a factor of two with one magnitude decrease in the front velocity.


 Please wait while we load your content...
Please wait while we load your content...