A mobile precursor determines protein resistance on nanostructured surfaces†
Abstract
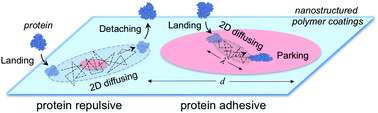
Biomaterials are often engineered with nanostructured surfaces to control interactions with proteins and thus regulate their biofunctions. However, the mechanism of how nanostructured surfaces resist or attract proteins together with the underlying design rules remains poorly understood at a molecular level, greatly limiting attempts to develop high-performance biomaterials and devices through the rational design of nanostructures. Here, we study the dynamics of nonspecific protein adsorption on block copolymer nanostructures of varying adhesive domain areas in a resistant matrix. Using surface plasmon resonance and single molecule tracking techniques, we show that weakly adsorbed proteins with two-dimensional diffusivity are critical precursors to protein resistance on nanostructured surfaces. The adhesive domain areas must be more than tens or hundreds of times those of the protein footprints to slow down the 2D-mobility of the precursor proteins for their irreversible adsorption. This precursor model can be used to quantitatively analyze the kinetics of nonspecific protein adsorption on nanostructured surfaces. Our method is applicable to precisely manipulate protein adsorption and resistance on various nanostructured surfaces, e.g., amphiphilic, low-surface-energy, and charged nanostructures, for the design of protein-compatible materials.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...