Small-angle X-ray scattering study on nano-scale structures controlled by water content in a binary water/ionic liquid system
Abstract
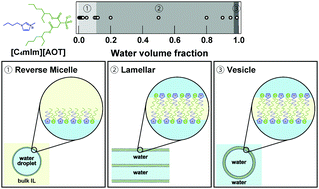
We report the water-in-ionic-liquid microemulsions (ME) formed in a binary water/ionic liquid system, without organic solvents, using a surfactant ionic liquid (SAIL) based on 1-butyl-3-methylimidazolium (C4mIm+) as the cation and dioctyl sulfosuccinate (AOT−) as the anion. Small-angle X-ray scattering (SAXS) revealed that MEs were stably formed in the binary water/SAIL solutions in the low water content region (water volume fraction, ϕw < 0.1), and the ME size systematically increased with increasing ϕw. We further investigated the nanostructures of the high ϕw region using a combination of SAXS and rheological measurements and found that the MEs changed to a stacked lamellar structure comprising SAIL bilayers and water phases at ϕw > 0.12. At the largest water content, ϕw = 0.99, vesicle structures were obtained.


 Please wait while we load your content...
Please wait while we load your content...