Photochemistry of glyoxylate embedded in sodium chloride clusters, a laboratory model for tropospheric sea-salt aerosols†
Abstract
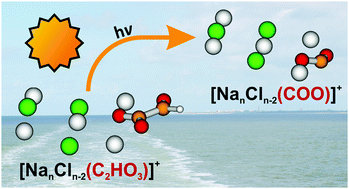
Although marine aerosols undergo extensive photochemical processing in the troposphere, a molecular level understanding of the elementary steps involved in these complex reaction sequences is still missing. As a defined laboratory model system, the photodissociation of sea salt clusters doped with glyoxylate, [NanCln−2(C2HO3)]+, n = 5–11, is studied by a combination of mass spectrometry, laser spectroscopy and ab initio calculations. Glyoxylate acts as a chromophore, absorbing light below 400 nm via two absorption bands centered at about 346 and 231 nm. Cluster fragmentation dominates, which corresponds to internal conversion of the excited state energy into vibrational modes of the electronic ground state and subsequent unimolecular dissociation. Photochemical dissociation pathways in electronically excited states include CO and HCO elimination, leading to [Nan−xCln−x−2HCOO]+ and [NanCln−2COO˙]+ with typical quantum yields in the range of 1–3% and 5–10%, respectively, for n = 5. The latter species contains CO2˙− stabilized by the salt environment. The comparison of different cluster sizes shows that the fragments containing a carbon dioxide radical anion appear in a broad spectral region of 310–380 nm. This suggests that the elusive CO2˙− species may be formed by natural processes in the troposphere. Based on the photochemical cross sections obtained here, the photolysis lifetime of glyoxylate in a dry marine aerosol is estimated as 10 h. Quantum chemical calculations show that dissociation along the C–C bond in glyoxylic acid as well as glyoxylate embedded in the salt cluster occurs after reaching the S1/S0 conical intersection, while this conical intersection is absent in free glyoxylate ions.


 Please wait while we load your content...
Please wait while we load your content...