Electronic structure and large second-order non-linear optical property of COT derivatives – a theoretical exploration†
Abstract
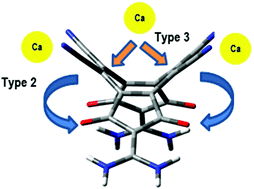
A new strategy to design new molecules based on a fused hydrocarbon ring system comprising a COT ring and two 5-membered rings has been proposed for the study of second order NLO properties. The four charge transferring groups –NR2 (R = H, Li, Na and K) in conjunction with a sufficient number of electron withdrawing groups lead to significant variation of structural parameters and polarity. The charge transfer characteristics can be strongly modulated by introducing calcium metal atoms at suitable sites. Ca metal atoms end-capping the nitrogen ends of two adjacent –CN groups lead to electride character while a Ca metal atom bonded directly to the COT ring leads to greater charge transfer. The size of the alkali metal atom has been found to have a dramatic effect on the enhancement of first hyperpolarizability. The most significant electronic asymmetry induced by the larger potassium metal atom strongly enhances the magnitude of first hyperpolarizability. The variation of first hyperpolarizability has been satisfactorily explained in terms of TD-CAMB3LYP calculated spectroscopic parameters in light of the two-state model.


 Please wait while we load your content...
Please wait while we load your content...