Adsorption and decomposition of dimethyl methylphosphonate on size-selected (MoO3)3 clusters†
Abstract
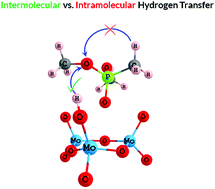
The adsorption and decomposition of dimethyl methylphosphonate (DMMP), a chemical warfare agent (CWA) simulant, on size-selected molybdenum oxide trimer clusters, i.e. (MoO3)3, was studied both experimentally and theoretically. X-ray photoelectron spectroscopy (XPS), temperature programmed reaction (TPR), and density functional theory (DFT)-based simulations were utilized in this study. The XPS and TPR results showed both, desorption of intact DMMP, and decomposition of DMMP through the elimination of methanol at elevated temperatures on (MoO3)3 clusters. Theoretical investigation of DMMP on (MoO3)3 clusters suggested that, in addition to pure (MoO3)3 clusters, reduced molybdenum oxide clusters and hydroxylated molybdenum oxide clusters also play an important role in decomposing DMMP via a “reverse Mars–van Krevelen mechanism”. The present study, which focused on oxide clusters, underlines the importance of surface defects, e.g., the oxygen vacancies and surface hydroxyls, in determining the reaction pathway of DMMP, in agreement with previous studies on thin films. In addition, the structural fluxionality and the Lewis acidity of molybdenum oxide clusters, i.e. (MoO3)3, may make them good candidates for adsorption and decomposition of chemical warfare agents with similar structures to DMMP.


 Please wait while we load your content...
Please wait while we load your content...